-
Engineer and label cells
-
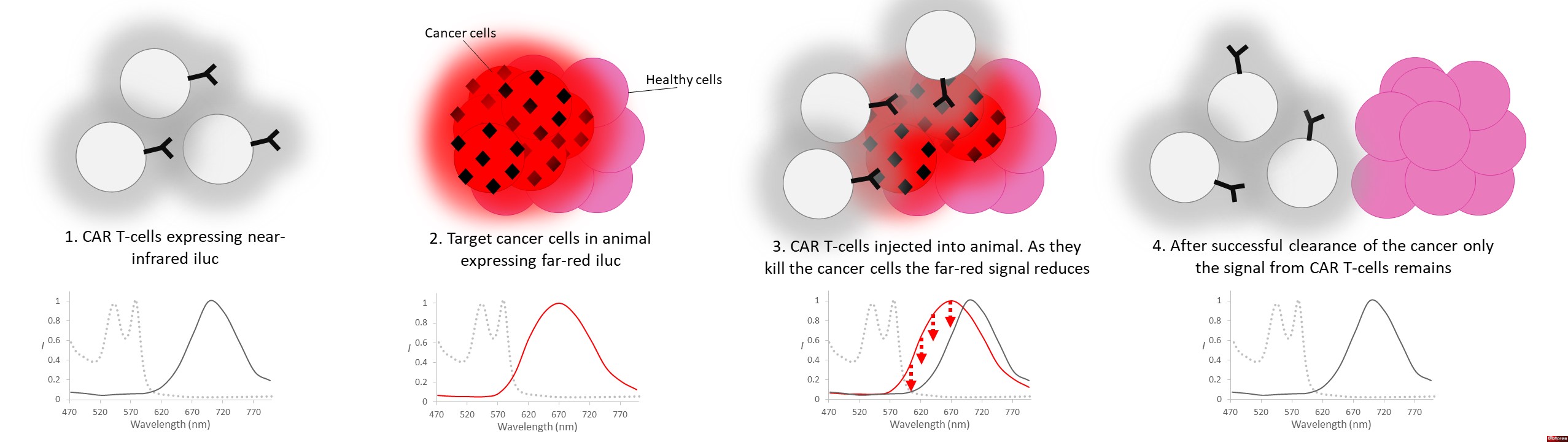
Tumor cells are engineered to express one variant of luciferase (e.g., red-shifted).
-
CAR T-cells are engineered to express a different luciferase variant (e.g., NIR-shifted).
-
-
Establish mouse model
-
Tumor cells are implanted into the mouse.
-
CAR T-cells are introduced (e.g., by infusion).
-
-
Administer Infraluciferin substrate
-
Bioflares’ Infraluciferin methyl ester is delivered systemically.
-
Once inside cells, esterases convert it into the active substrate.
-
-
Capture dual bioluminescence signals
-
Tumor cells and CAR T-cells emit distinct NIR signals.
-
Imaging systems detect and quantify both signals non-invasively.
-
-
Analyze therapeutic response
-
Monitor CAR T-cell expansion, persistence, and trafficking.
-
Track tumor regression (or relapse) in the same animal.
-
Assess safety features such as CAR T-cell “suicide switches.”
-
Advantages for CAR T-cell Studies
-
Real-time efficacy assessment – Quantify tumor killing by CAR T-cells dynamically in living animals (Stowe et al., 2019).
-
Superior accuracy – Far-red to NIR imaging penetrates deeper, improving quantification and resolution.
-
Ethical and cost benefits – Dual imaging can halve animal usage by combining readouts into a single study.
-
Safety validation – A powerful way to test built-in CAR safety mechanisms in vivo.
Summary
By combining Infraluciferin’s color-shifting properties with engineered luciferase variants (iLuc), dual NIR bioluminescence provides an unprecedented window into CAR T-cell therapy. Researchers can track tumor burden and immune activity together—non-invasively, longitudinally, and with high sensitivity—accelerating preclinical testing and improving translational outcomes.